
South Korean biotechnology enterprises are aiming at the $26 billion biosimilar sector which is expanding due to over ten major drug patents expiring in the U.S. this year. These biosimilars refer to replicas of initial biological medications produced once their patent terms conclude. Capitalizing on this fresh prospect within the globe’s biggest healthcare marketplace, South Korean medicinal corporations are rolling out their versions of these biosimilars for competitive entry into the field.
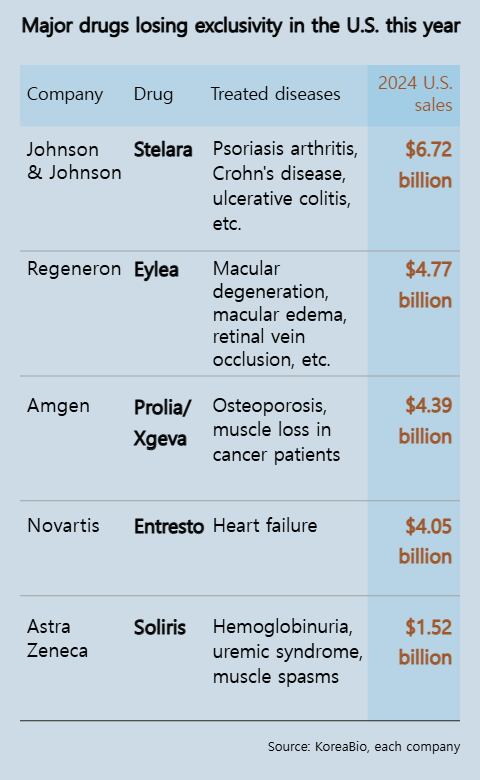
As reported by the Korea Biotechnology Industry Organization (KoreaBio) referencing Fierce Pharma, the total revenue from the top 10 blockbuster medications losing or scheduled to lose their exclusive rights in the United States amounted to $26.2 billion (approximately 38.2 trillion Korean won) in sales for the previous year. These figures were derived from pharmaceutical profit statements and information within the medical sector.
Among the prominent medications with lapsed patents, Johnson & Johnson’s autoimmunity therapy Stelara topped U.S. sales for the previous year. Globally, Stelara generated $10.36 billion in revenues, out of which $6.72 billion were earned within the United States. This amount exceeds the total yearly income of South Korea’s leading ten pharmaceutical and biotechnology firms.
With Stelara’s patent expiring last year, many global drugmakers have been developing biosimilars. Seven are already competing in the U.S. market, with more expected to launch soon.
Major South Korean drug companies are joining the competition to develop biosimilars of Stelara. Samsung Bioepis took the lead by introducing Pyzchiva in the United States on February 24. Additionally, both Celltrion and Dong-A ST have received FDA approval for their respective products, Steqeyma and Imuldosa, with plans to release them later this year.
Samsung Bioepis and Celltrion are preparing to introduce their biosimilar versions of osteoporosis treatments in the United States. These new products aim at competing with Amgen’s well-established medications, Prolia and Xgeva, which are commonly prescribed for osteoporosis and bone cancer. In the previous year, these two drugs collectively earned $6.6 billion globally, out of which $4.39 billion was realized within the American market.
When major pharmaceutical patent expirations occur, they present significant opportunities for South Korean biotechnology firms that specialize in developing biosimilars. These biosimilars offer comparable effectiveness at reduced costs, resonating well with U.S. healthcare strategies intended to decrease medication expenses. As reported by KoreaBio’s Bio-Economic Research Center, between 2015 and early this year, the FDA had authorized 68 biosimilars within the United States. Among them, twenty were produced in South Korea, positioning it as the world's second-biggest producer right behind the U.S., home to 26 such products.
South Korean enterprises are outperforming in the biosimilar sector by expediting clinical trials compared to international counterparts. Diverging from generics, which can gain approval simply through duplicating the chemical makeup of the reference product without undergoing clinical tests, biosimilars necessitate proof via clinical data demonstrating identical biological characteristics, thus complicating their development process. The concluding phase—Phase 3 trials—which are notably lengthy, pose significant challenges; however, South Korea’s biotechnology corporations accomplish this stage up to one year quicker than competing foreign entities, as reported by the American database ClinicalTrials.gov.


Post a Comment